
|
An artist's impression of the giant graphite crystal that visitors passed through on entry. (See The Story the Exhibition Tells) | ||
|
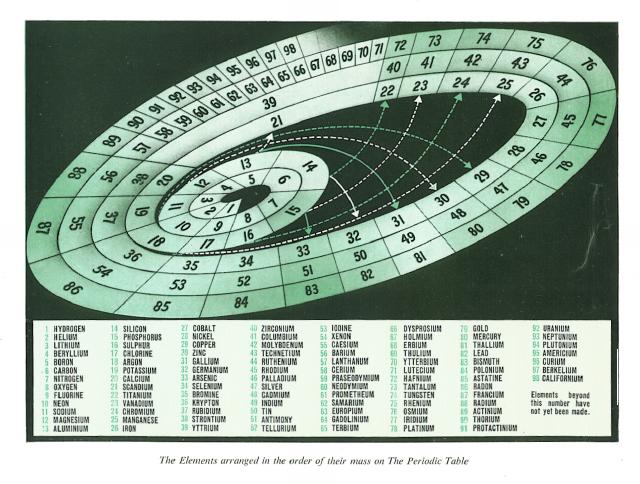
The exhibition begins by looking at the nature and structure of matter. At the outset we see what are the elements of which everything around us is made. A table shows these elements arranged in their order, the lightest atoms first and the heaviest last. When this is done, the elements which are similar in their use and action form regular groups. The great cycle of these groups is the Periodic Table. But the Periodic Table is not yet closed, because man is still creating new elements which are heavier than any made by nature. |
||

|
Elements and atoms are all very well, but how do we get to these in the first place from the robust pots and pans which we handle in everyday life? Take a nice homely object like a fish-slice. We detach the blade and there we have an element; for the blade is made of aluminium and aluminium is an element — it cannot be separated into other chemical parts. The handle is made of wood. If we heat it slowly we drive off the water in its fibres and are left at last with a sooty char which, for the most part, is another element, carbon. As for the water, an electric current will break that into its elements, the gases oxygen and hydrogen. There are many methods of this kind in chemistry and physics. Some clean a substance of its impurities. Others separate a mixture of substances into different parts. And others finally break down a substance into the elements from which it is compounded. This is how, step by step, we discover the elements within all the natural and the man-made materials which we meet in the world. |
||